Preparation and Electrochemical Capacitance Performance of MnFePBA/rGO/CC Composite Electrode
-
摘要:
采用共沉淀法在氧化石墨烯(GO)表面原位生长锰铁普鲁士蓝类似物(MnFePBA)纳米颗粒,获得不同MnFePBA和GO质量比(1∶0.1,1∶0.3,1∶0.5)的复合粉体;采用超声喷涂法将MnFePBA/GO复合粉体涂敷在预热的碳布(CC)基底上,并借助化学还原将GO转化成还原氧化石墨烯(rGO),制备出MnFePBA/rGO/CC复合电极,研究了复合电极的微观结构和电化学电容性能。结果表明:当MnFePBA与GO的质量比为1∶0.1时,MnFePBA颗粒发生团聚;当二者的质量比为1∶0.5时,GO纳米片出现明显堆叠;当二者的质量比为1∶0.3时,GO与MnFePBA均匀复合,所制备的MnFePBA/GO/CC复合电极具有最高的比电容、最小的内阻及最快的离子扩散速率,电化学性能最优。当MnFePBA和GO质量比为1∶0.3时,化学还原法制备的MnFePBA/rGO/CC复合电极在1 A·g-1电流密度下的比电容由化学还原前的888 F·g-1增加到1 032 F·g-1;当电流密度从1 A·g-1增大到10 A·g-1时,比电容保持率由化学还原前的44.93%提升至54.55%,且在7 A·g-1电流密度下经过3 000圈循环后的比电容保持率仍为94.78%。
Abstract:MnFe-Prussian blue analog (MnFePBA) nanocubes were in-situ grown on graphene oxide (GO) surface by co-precipitation. The composite powders with different MnFePBA to GO mass ratios (1:0.1, 1:0.3, 1:0.5) were prepared, and then ultrasonically sprayed onto preheated carbon cloth (CC) substrate. The MnFePBA/rGO/CC composite electrode was prepared by converting GO to reduced graphene oxide (rGO) by chemical reduction. The microstructure and electrochemical capacitance performance of the composite electrode were investigated. The results show that when the mass ratio of MnFePBA to GO was 1: 0.1, agglomeration of MnFePBA particles occurred. When the mass ratio was 1:0.5, obvious stacking of GO nanoflakes was found. With the MnFePBA to GO mass ratio of 1:0.3, uniform hybridization of GO and MnFePBA was achieved, and the as-prepared MnFePBA/GO/CC composite electrode exhibited the best electrochemical performance with the largest specific capacitance, the smallest internal resistance and the largest ion diffusion rate. When the mass ratio of MnFePBA to GO was 1:0.3, the specific capacitance at 1 A·g-1 of MnFePBA/rGO/CC composite electrode prepared by chemical reduction increased from 888 F·g-1 before chemical reduction to 1 032 F·g-1. As the current density increased from 1 A·g-1 to 10 A·g-1, the specific capacitance retention was enhanced from 44.93% before chemical reduction to 54.55%. After cycling 3 000 cycles at 7 A·g-1, the MnFePBA/rGO/CC composite electrode still maintained 94.78% of specific capacitance retention.
-
0. 引言
随着化石燃料的快速消耗,环境污染与能源危机等问题也日益严重,太阳能、风能、潮汐能等可再生能源引起了人们的广泛关注[1]。但是,这些可再生能源产生电能的稳定性受地理环境的影响很大,因此,低成本、高效且可靠的能源存储器件成为近年来国内外科研工作者研究的重点。超级电容器作为一种可以快速存储、供应高功率电力并具有长循环寿命的电化学储能器件,引起了研究者的广泛关注[2]。超级电容器根据电荷存储机理的不同主要分为双电层电容器与赝电容器。双电层电容器的电极材料主要以碳点(CDs)、石墨烯、碳纳米纤维(CNF)和多孔碳等碳材料为主,具有优异的导电性,但较低的比电容以及能量密度限制了其应用领域。相比之下,赝电容器具有较高的理论比容量和能量密度,应用较广泛,其主要电极材料包括过渡金属氧化物、导电聚合物以及普鲁士蓝类似物(PBA)[3-4]等,但是这些赝电容电极材料的导电性较差,往往需要与碳材料相复合以改善其导电能力。
PBA由于具有开放的三维框架结构、简单的合成方法以及可调的活性中心等优点而被认为是一种优异的电极材料。PBA的化学式一般表示为A x M1[M2(CN)6]y·n H2O,其中:A为碱金属元素,包括锂、钠或钾;M1表示与氮配位的过渡金属元素,一般为铁、钴、镍、铜、锌等;M2表示与碳配位的过渡金属元素,一般为铁、钴、锰等。PBA的电化学性质可以通过调节不同的过渡金属元素来控制[5]。然而,PBA的电导率低,不利于电荷的转移[6],需要将其与碳点[7]、碳纳米管[8]和石墨烯[9]等高导电的碳材料复合来改善其导电能力。例如:GUO等[10]将镍铁普鲁士蓝类似物(NiFePBA)与碳点复合,采用原位沉淀法在泡沫镍(NF)表面生长CDs/NiFePBA纳米颗粒,所制备的CDs/NiFePBA纳米立方体/NF复合电极表现出高的比电容与优异的循环稳定性。在各种PBA材料中,锰铁普鲁士蓝类似物(MnFePBA)由于成本低廉、易于制备、金属锰储量丰富等优点而受到关注[11],但其实际应用往往受到电导率低及循环稳定性不佳等缺点的制约。为此,作者通过共沉淀法在氧化石墨烯(GO)表面原位生长MnFePBA纳米颗粒,并借助超声喷涂技术将MnFePBA/GO复合粉体涂敷在碳布(CC)表面,然后通过化学还原将GO转变为还原氧化石墨烯(rGO),制备出无黏结剂的MnFePBA/rGO/CC复合电极,研究了MnFePBA与GO质量比对复合电极结构、形貌及电化学电容性能的影响,以期为PBA复合电极材料的制备提供试验参考。
1. 试样制备与试验方法
试验原料:四水合硝酸锰Mn(NO3)2·4H2O、铁氰化钾K3[Fe(CN)6]、七水合硫酸锌ZnSO4·7H2O、一水合硫酸锰MnSO4·H2O和抗坏血酸L(+)-Ascorbic acid,均为分析纯,由上海泰坦科技有限公司提供;氧化石墨烯浆料(LN-10R,质量分数1%),由上海利物盛纳米科技有限公司提供;碳布(WOS1011),由苏州晟尔诺科技有限公司提供。
对碳布进行亲水处理[12],具体流程:将碳布置于三口烧瓶中,将浓硝酸与浓硫酸按体积比3∶1混合均匀后,倒入三口烧瓶并浸没碳布,在120 ℃下冷凝回流2 h酸化;酸化后的碳布用去离子水冲洗3次,再放置在盛有去离子水的烧杯中超声振荡20 min,以充分去除碳布上残留的硫酸和硝酸,然后用去离子水漂洗至中性;将洗净的碳布置于60 ℃的鼓风干燥箱过夜烘干。
制备MnFePBA/GO粉体,具体流程:称取0.2 mmol K3[Fe(CN)6]加入到100 mL的GO浆料中,磁力搅拌得到均匀溶液A;称取2 mmol Mn(NO3)2·4H2O溶于100 mL去离子水中,磁力搅拌得到均匀溶液B;在室温下通过注射泵将溶液B以5 mL·min-1的流量滴加至溶液A中,并在500 r·min-1的转速下磁力搅拌90 min,再在室温下静置24 h;经离心、洗涤去除杂质,再于-40 ℃下冷冻干燥24 h得到MnFePBA/GO粉体。为了获得MnFePBA与GO的最佳质量比,控制Fe(CN)36-、Mn2+加入量不变,即MnFePBA的质量为58.8 mg(0.1 mmol),调节GO浆液的质量浓度分别为0.058 8,0.176 4,0.294 0 mg·mL-1,即对应于MnFePBA与GO的质量比分别为1∶0.1,1∶0.3,1∶0.5制备MnFePBA/GO粉体,分析其性能以及制成电极的性能。将制备的MnFePBA/GO复合粉体分别命名为MnFePBA/GO-0.1粉体、MnFePBA/GO-0.3粉体和MnFePBA/GO-0.5粉体。
制备MnFePBA/GO/CC和MnFePBA/rGO/CC复合电极。称取15 mg上述MnFePBA/GO粉体超声分散于50 mL去离子水中,配制成0.3 mg·mL-1的悬浮液。采用UC320型超声喷涂设备制备复合电极。将亲水处理后的碳布裁剪成尺寸为1 cm×1 cm的小块碳布,然后置于预热至200 ℃的加热台固定位置。超声喷涂时的注射器进液流量设置为0.3 mL·min-1,超声功率为1.5 W,载气压力为0.15 MPa。悬浮液经过超声雾化后,采用步进扫描方式(扫描速度2 mm·s-1)将雾化液滴喷涂在预热的碳布上,溶剂在高温下挥发形成活性物质,并附着在碳布纤维的表面。将超声喷涂所制备的MnFePBA与GO质量比为1∶0.1,1∶0.3,1∶0.5的复合电极分别命名为MnFePBA/GO/CC-0.1、MnFePBA/GO/CC-0.3、MnFePBA/GO/CC-0.5。为了进一步增强GO的导电性,将MnFePBA/GO/CC-0.3复合电极浸泡在1 mg·mL-1的抗坏血酸溶液中,在90 ℃水浴中还原处理15 min,经去离子水漂洗后放入鼓风干燥箱中于60 ℃干燥8 h,即得到MnFePBA/rGO/CC-0.3复合电极。
采用Bruker D8-Advance型X射线衍射仪(XRD)分析复合材料的物相组成和晶体结构,采用铜靶Kα射线,扫描速率为3(°)·min-1。通过Labram HR800 EVo型拉曼光谱仪(Raman)和SPE CTRUM 100型傅里叶变换红外光谱仪(FT-IR)分析复合材料的结构。选用Quanta FEG450型扫描电子显微镜(SEM)观察微观形貌,采用SEM附带的能谱仪(EDS)进行元素面扫描分析。使用Gamry INTERFACE 1010E型电化学工作站在标准三电极系统中进行电化学测试,复合电极为工作电极,铂片为对电极,饱和甘汞(SCE)为参比电极。工作电极面积为1 cm×1 cm,电解液为1 mol·L-1 ZnSO4+0.1 mol·L-1 MnSO4溶液。循环伏安(CV)测试的电压范围为0~0.9 V,扫描速率为5,10,20,30,40,50 mV·s-1。在0~0.9 V电压范围和不同电流密度(1~10 A·g-1)下进行恒电流充放电(GCD)测试。电化学阻抗谱(EIS)测试的频率范围为0.01 Hz~100 kHz。通过GCD测试得到放电时间,计算比电容,计算公式[13-14]如下:

(1) 式中:C为电极的比电容,F·g-1;I为放电电流,A;ΔU为电势窗口,V;m为活性物质的负载量,g;Δt为放电时间,s。
2. 试验结果与讨论
2.1 不同MnFePBA和GO质量比下的结构与电化学电容性能
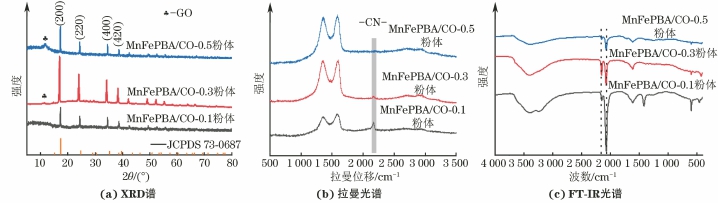
由图1可知,3种粉体在2θ为17.5°,24.8°,35.4°,39.8°的衍射峰分别对应于PBA的(200)、(220)、(400)和(420)晶面(JCPDS 73-0687),证明其为面心立方结构,同时PBA的衍射峰均表现出较高的强度与较小的半峰宽,说明复合材料中PBA具有较高的结晶程度[15]。MnFePBA/GO-0.3和MnFePBA/GO-0.5复合粉体在2θ为11.7°处观察到明显的GO衍射峰。3种粉体的拉曼光谱中均出现了石墨烯的特征D带和G带,其中1 350 cm-1处的D峰来自于石墨烯中的缺陷,而1 580 cm-1处的G峰则对应于sp2结构的碳原子振动[16];在2 160 cm-1处的振动信号归因于Fe(Ⅲ)-CN-Mn(Ⅱ)的特征峰[17]。3种粉体的FT-IR光谱在波数3 364,1 609 cm-1左右出现的吸收峰来源于H2O中O-H的拉伸振动与弯曲振动,这归因于MnFePBA框架中的水分子振动[18];在2 149,2 070 cm-1处的吸收峰归因于PBA中氰基(-CN-)的振动,分别对应Fe(Ⅲ)-CN-Mn与Fe(Ⅱ)-CN-Mn[19];此外,593,450 cm-1处的吸收峰分别归因于Fe-CN的拉伸振动与弯曲振动。上述分析表明成功合成出了MnFePBA/GO复合粉体。
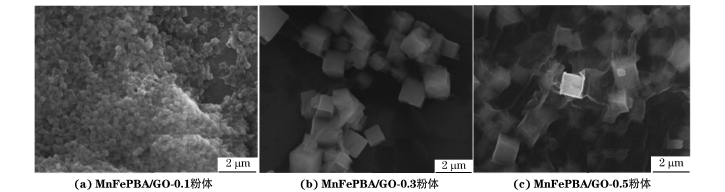
由图2可见,随着MnFePBA与GO质量比的降低,MnFePBA/GO复合粉体中GO纳米片逐渐增多,PBA颗粒的数量减少,但尺寸显著增大。当MnFePBA与GO的质量比为1∶0.1时,MnFePBA颗粒尺寸较小,但此时GO加入量较少,无法有效抑制MnFePBA的团聚;当MnFePBA与GO的质量比为1∶0.5时,复合粉体中的GO出现一定程度的堆叠,表明GO的加入量过多。当MnFePBA与GO的质量比为1∶0.3时,MnFePBA被GO纳米片均匀包覆,即实现了二者的均匀复合。
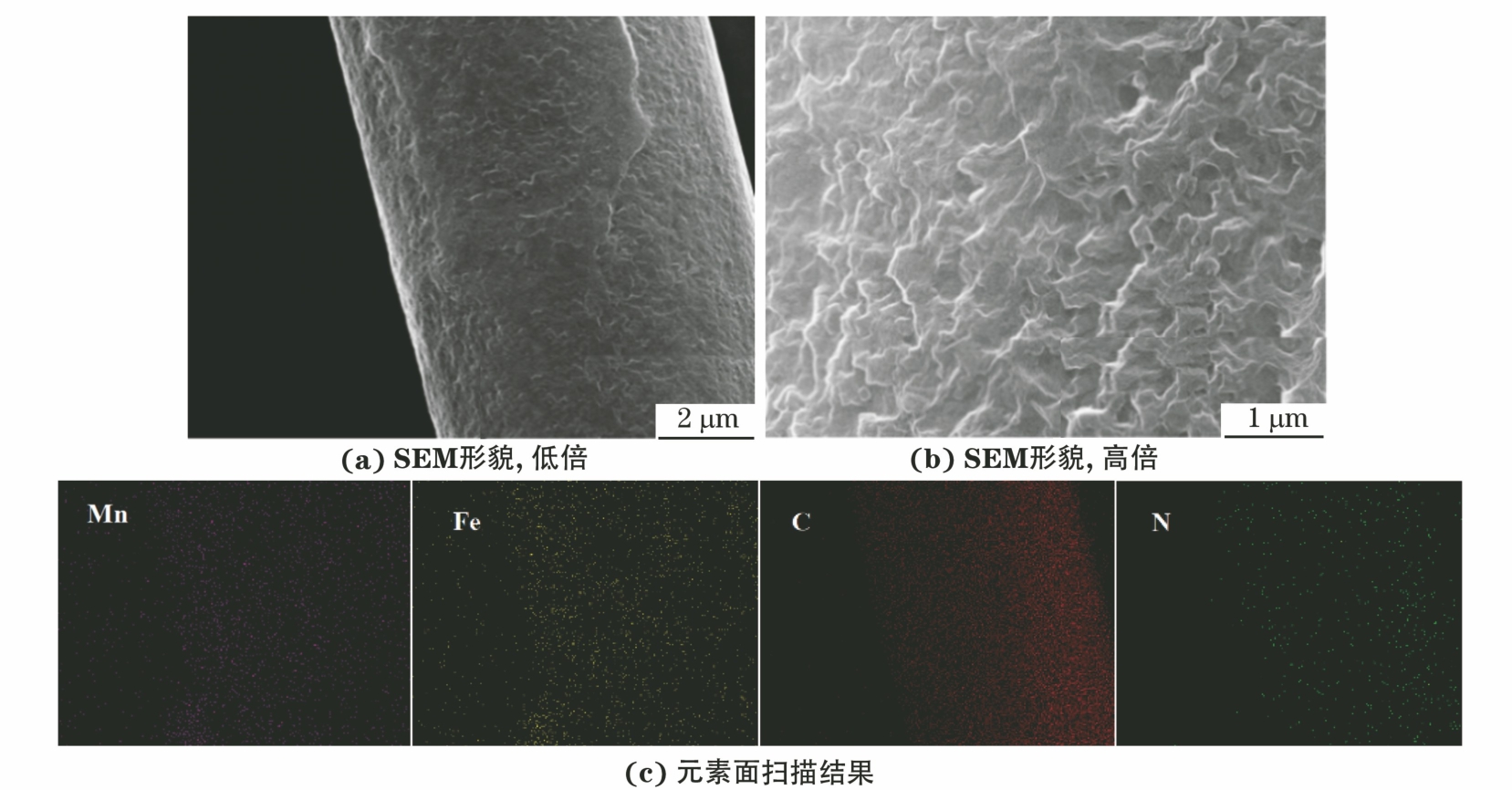
由图3可以看出,MnFePBA/rGO/CC-0.3复合电极中的MnFePBA颗粒被rGO纳米片包裹并紧密沉积在碳布纤维表面。锰、铁、碳、氮等元素均匀地分散在碳布纤维表面,表明MnFePBA在rGO表面均匀生长。
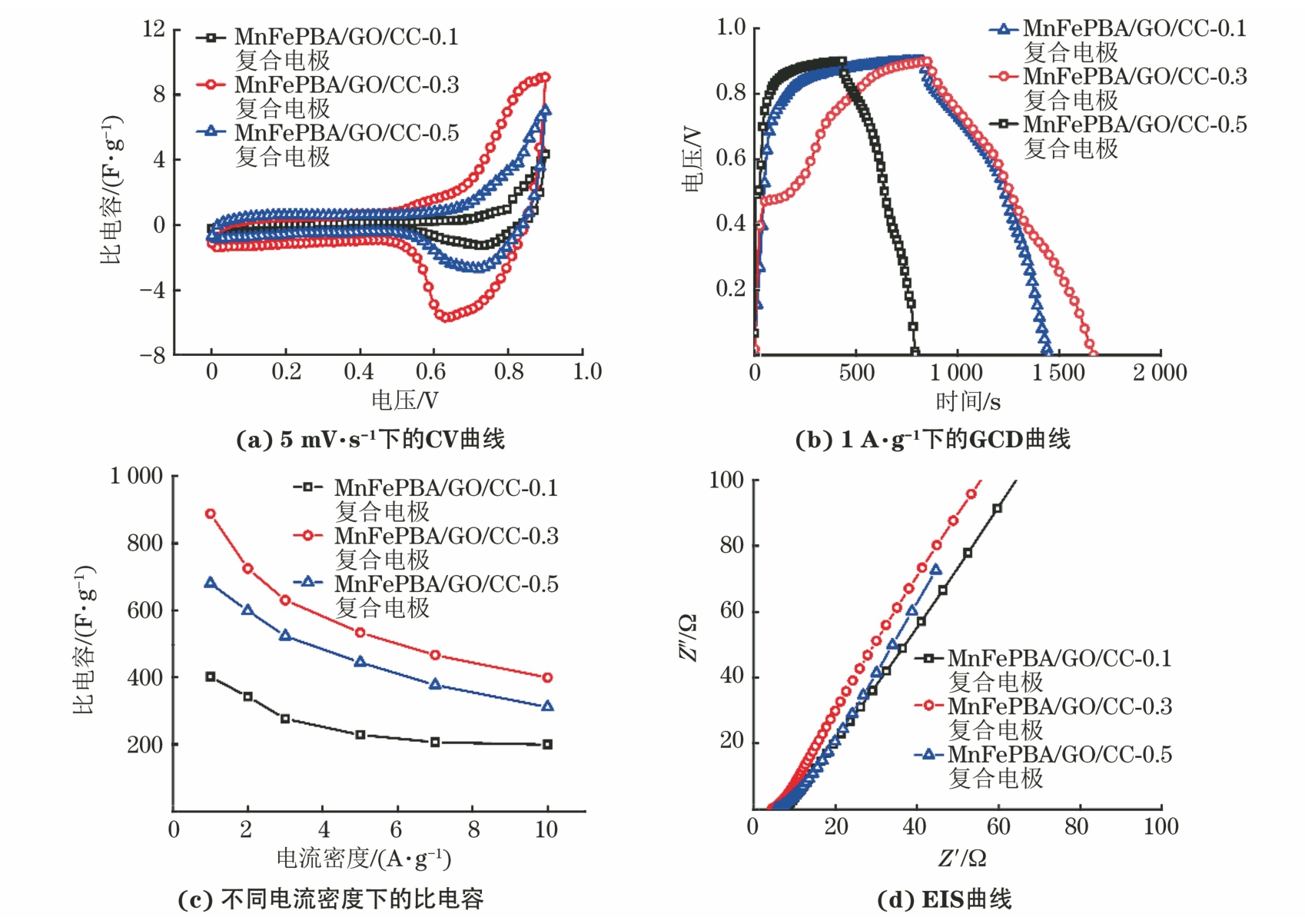
由图4可以看出,在5 mV·s-1扫描速率下,MnFePBA/GO/CC-0.1、MnFePBA/GO/CC-0.3和MnFePBA/GO/CC-0.5复合电极的CV曲线中均存在一对明显的氧化还原峰,对应于Mn3+/Mn2+的氧化还原反应。其中,MnFePBA/GO/CC-0.3复合电极的CV曲线包围的面积最大,说明该复合电极具有最高的比电容。MnFePBA/GO/CC-0.3复合电极的放电时间最长,且在1~10 A·g-1电流密度下均保持最高的比电容。在1 A·g-1电流密度下,MnFePBA/GO/CC-0.3复合电极的比电容为888 F·g-1,而当电流密度增大10倍后,比电容保持率为44.93%,表现出较好的倍率性能。MnFePBA/GO/CC-0.3复合电极的EIS曲线在高频区与实轴的截距最小,低频区的曲线斜率最大,这表明该复合电极拥有最低的等效串联电阻Rs和最小的Warburg阻抗。此外,所有复合电极在高频区均未发现明显的半圆弧,说明电极/电解质界面处电荷转移电阻Rct极小[10,20]。复合电极中离子扩散系数公式[20]为

(2) 式中:R为气体常数,8.314 J·mol-1·K-1;T为热力学温度,298 K;A为工作电极的表面积,1 cm2;n为反应电子数;F为法拉第常数,96 500 C·mol-1;C为离子浓度;σ为Warburg系数,即EIS曲线低频区的斜率。
对低频区的EIS曲线进行拟合,计算得到MnFePBA/GO/CC-0.1、MnFePBA/GO/CC-0.3和MnFePBA/GO/CC-0.5复合电极的锌离子扩散系数分别为1.19×10-11,5.72×10-11,1.73×10-11 cm2·s-1。更小的内阻及更快的离子扩散速率使得MnFePBA/GO/CC-0.3复合电极表现出最优的电化学性能。
2.2 还原前后结构与电化学电容性能的对比
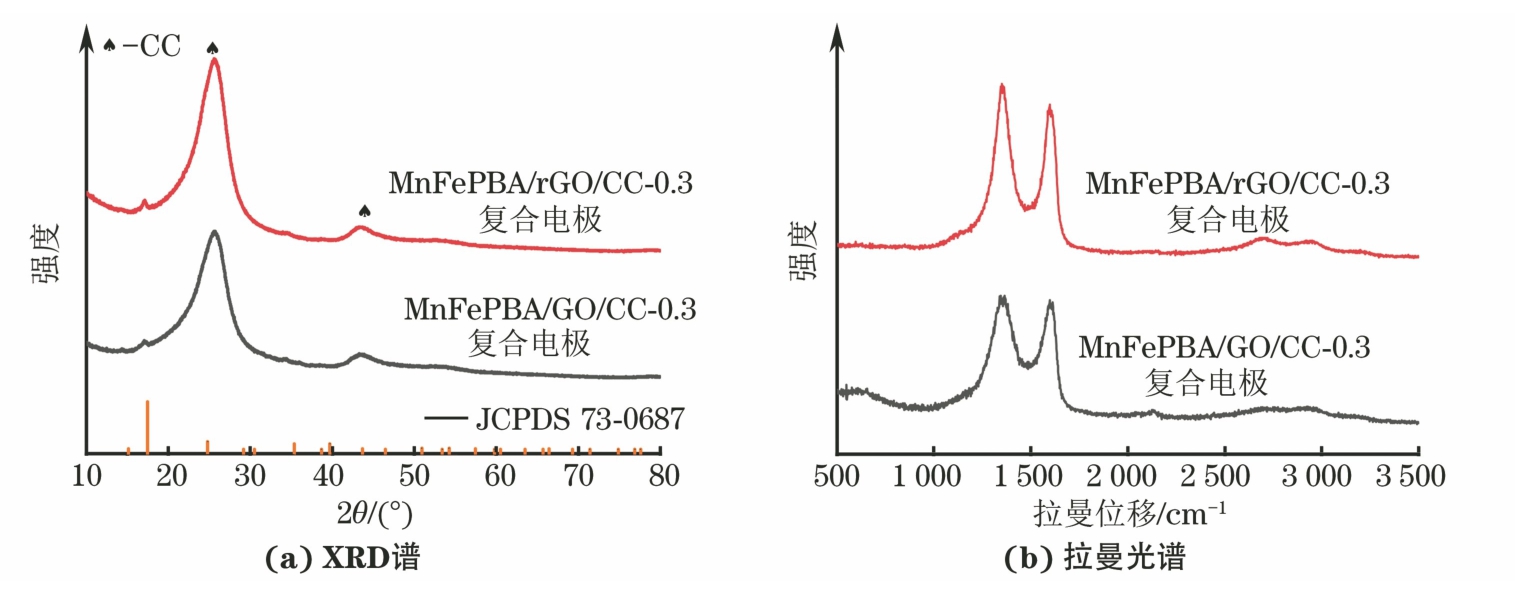
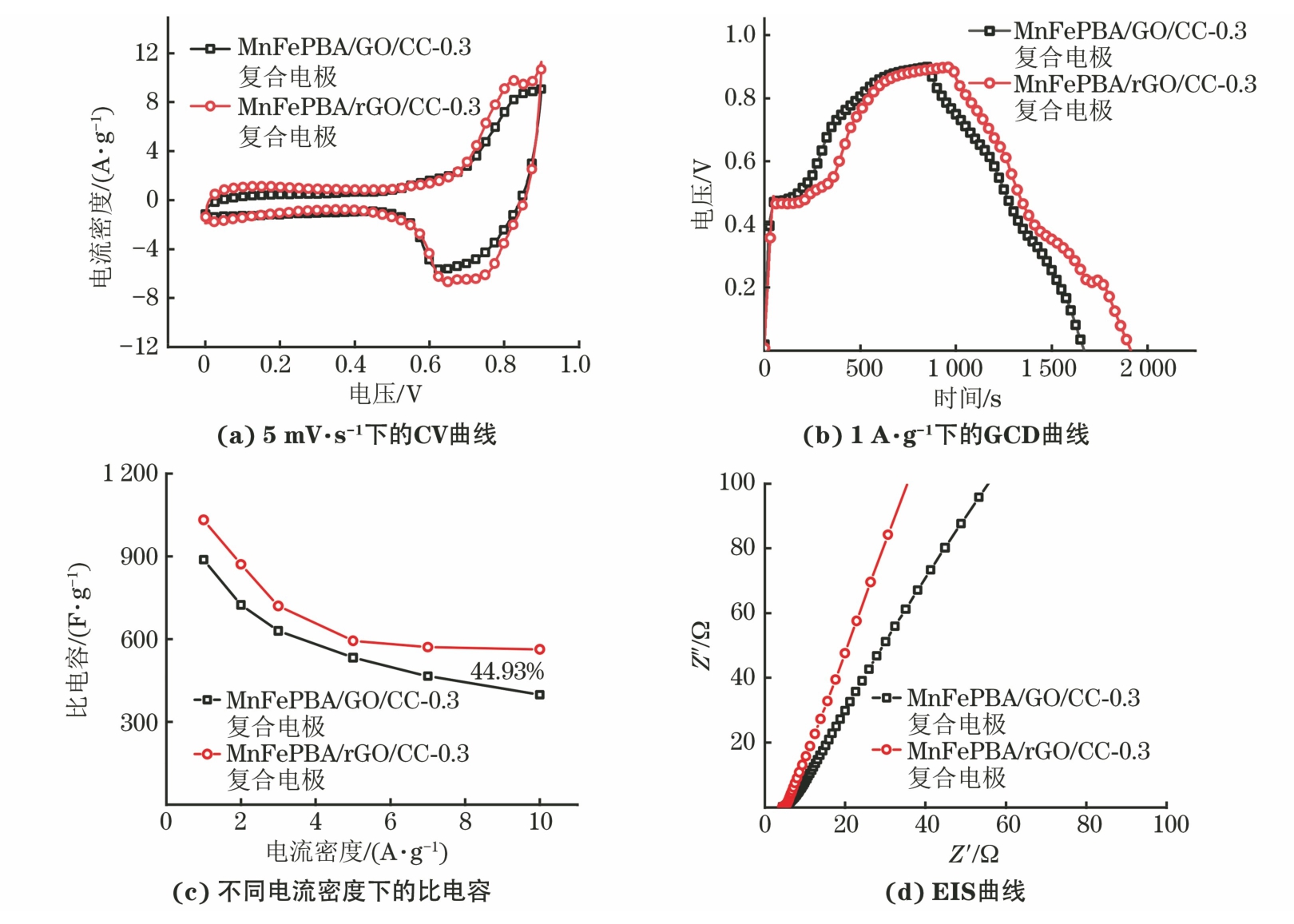
由图5可以看出,化学还原处理前后复合电极的XRD谱相似。由于MnFePBA在碳布表面的负载量较低,因此MnFePBA/GO/CC-0.3和MnFePBA/rGO/CC-0.3复合电极均仅在2θ为17.5°和35.4°处检测出PBA的衍射峰,而2θ为26°和43°处的衍射峰主要来源于基底碳布。通过拉曼光谱中石墨烯特征D带和G带的面积比(ID/IG)来判断材料的缺陷程度[21]。经化学还原处理后的ID/IG由处理前的2.06降低至1.74,表明GO表面部分含氧官能团得到消除,GO成功还原为rGO。
由图6可以发现:MnFePBA/rGO/CC-0.3复合电极的CV曲线包围的面积更大,说明比电容更高,在0.73 V处出现的氧化峰归因于Zn2+的嵌入,表明rGO的存在加速了电荷的转移;还原后的MnFePBA/rGO/CC-0.3复合电极具有更长的放电时间,说明该电极具有更大的比电容,同时在0.21 V处出现新的放电平台,可能是由于MnFePBA中铁的激活[22]。MnFePBA/rGO/CC-0.3复合电极在1 A·g-1电流密度下的比电容高达1 032 F·g-1,当电流密度从1 A·g-1增大到10 A·g-1时,比电容保持率由化学还原前的44.93%提升至54.55%,说明化学还原后复合电极的倍率性能得到明显提升。2个复合电极的Rs与Rct均无较大变化,而化学还原后复合电极的低频区Warburg阻抗显著减小。根据EIS拟合计算结果,经化学还原处理后的MnFePBA/rGO/CC-0.3复合电极的锌离子扩散系数得到进一步提升,其值为5.72×10-11 cm2·s-1。因此,MnFePBA/rGO/CC-0.3复合电极的电化学性能优于MnFePBA/GO/CC-0.3复合电极。
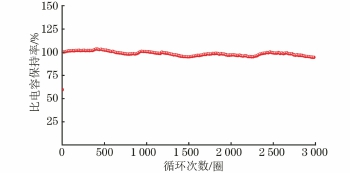
由图7可以看出:在7 A·g-1电流密度下MnFePBA/rGO/CC-0.3复合电极经过3 000圈循环后的比电容保持率为94.78%,这说明在测试过程中,电极表面的MnFePBA/rGO活性物质未发生明显的脱落;比电容保持率的轻微起伏是由测试过程中温度的波动引起的。因此可知,MnFePBA/rGO/CC-0.3复合电极表现出优异的循环稳定性。
MnFePBA/rGO/CC-0.3复合电极优异的电化学电容性能与其结构特点密切相关:(1)rGO的存在除了能提升材料的导电性外,还能有效抑制PBA颗粒的团聚,促进电解质离子的扩散;(2)PBA颗粒在GO表面的原位生长,有利于获得更优的界面接触性能,从而加快界面电子转移;(3)超声喷涂法制备的复合电极中的活性物质与碳布基底间结合紧密,有利于获得更优异的循环稳定性。此外,电化学测试中选用ZnSO4与MnSO4的混合电解液,可以在一定程度上缓解锰的溶解,这有利于改善电极材料的循环稳定性。
3. 结论
(1)当MnFePBA与GO质量比为1∶0.1时,较少的GO无法抑制MnFePBA/GO复合粉体中MnFePBA的团聚;当MnFePBA与GO质量比为1∶0.5时,过多的GO纳米片发生堆叠;当MnFePBA与GO质量比为1∶0.3时,GO与MnFePBA均匀复合,此时采用超声喷涂技术制备的MnFePBA/GO/CC复合电极具有最高的比电容,最小的内阻以及最快的离子扩散速率,电化学性能最优。
(2)当MnFePBA与GO的质量比为1∶0.3时,通过化学还原法得到的MnFePBA/rGO/CC复合电极在1 A·g-1电流密度下的比电容由还原前的888 F·g-1增加到1 032 F·g-1;当电流密度从1 A·g-1增大到10 A·g-1时,比电容保持率由还原前的44.93%提升至54.55%,在7 A·g-1电流密度下经过3 000圈循环后的比电容保持率仍达94.78%。
-
-
[1] NIU L, WU T Z, CHEN M, et al. Conductive metal-organic frameworks for supercapacitors[J]. Advanced Materials, 2022, 34(52): e2200999. [2] DONG W J, XIE M, ZHAO S W, et al. Materials design and preparation for high energy density and high power density electrochemical supercapacitors[J]. Materials Science and Engineering: R, 2023, 152: 100713. [3] GONZÁLEZ A, GOIKOLEA E, BARRENA J A, et al. Review on supercapacitors: Technologies and materials[J]. Renewable and Sustainable Energy Reviews, 2016, 58: 1189-1206. [4] CHEN J S, WEI L, MAHMOOD A, et al. Prussian blue, its analogues and their derived materials for electrochemical energy storage and conversion[J]. Energy Storage Materials, 2020, 25: 585-612. [5] XU C W, YANG Z W, ZHANG X K, et al. Prussian blue analogues in aqueous batteries and desalination batteries[J]. Nano-Micro Letters, 2021, 13(1): 166. [6] WANG J G, REN L B, HOU Z D, et al. Flexible reduced graphene oxide/Prussian blue films for hybrid supercapacitors[J]. Chemical Engineering Journal, 2020, 397: 125521. [7] SONG Z X, LIU W, ZHOU Q, et al. Coordination-induced activation of reversible Co(II)/Co(III) redox reaction in carbon nanodots/cobalt hexacyanoferrate composites with enhanced electrochemical performance[J]. Journal of Alloys and Compounds, 2022, 893: 162368. [8] XU P P, WANG G L, WANG H H, et al. K2.25Ni0.55Co0.37Fe(CN)6 nanoparticle connected by cross-linked carbon nanotubes conductive skeletons for high-performance energy storage[J]. Chemical Engineering Journal, 2017, 328: 834-843. [9] HE Y F, ZHANG P P, WANG M, et al. Nano-sandwiched metal hexacyanoferrate/graphene hybrid thin films for in-plane asymmetric micro-supercapacitors with ultrahigh energy density[J]. Materials Horizons, 2019, 6(5): 1041-1049. [10] GUO Z R, SONG R B, ZHANG L, et al. Three-dimensional carbon dots/Prussian blue analogues nanocubes/nickel foams as self-standing electrodes for high-performance hybrid electrochemical capacitors[J]. Journal of Colloid and Interface Science, 2022, 613: 796-805. [11] KAZAZI M, FARYABI M. Electrochemically anchored manganese hexacyanoferrate nanocubes on three-dimensional porous graphene scaffold: Towards a potential application in high-performance asymmetric supercapacitors[J]. Journal of Power Sources, 2020, 449: 227510. [12] ZHANG S S, GUO M J, SONG S Y, et al. Hierarchical Mo-doped CoP3 interconnected nanosheet arrays on carbon cloth as an efficient bifunctional electrocatalyst for water splitting in an alkaline electrolyte[J]. Dalton Transactions, 2020, 49(17): 5563-5572. [13] SHI Z T, SUN G, YUAN R W, et al. Scalable fabrication of NiCo2O4/reduced graphene oxide composites by ultrasonic spray as binder-free electrodes for supercapacitors with ultralong lifetime[J]. Journal of Materials Science & Technology, 2022, 99: 260-269. [14] SENTHILKUMAR S T, KIM J, WANG Y, et al. Flexible and wearable fiber shaped high voltage supercapacitors based on copper hexacyanoferrate and porous carbon coated carbon fiber electrodes[J]. Journal of Materials Chemistry A, 2016, 4(13): 4934-4940. [15] LIANG J, TIAN B, LI S Q, et al. All-printed MnHCF-MnOx-based high-performance flexible supercapacitors[J]. Advanced Energy Materials, 2020, 10(12): 2000022. [16] MENDES L F, DE SIERVO A, REIS DE ARAUJO W, et al. Reagentless fabrication of a porous graphene-like electrochemical device from phenolic paper using laser-scribing[J]. Carbon, 2020, 159: 110-118. [17] SUEMOTO T, OHKI K, FUKAYA R, et al. Dynamics of the charge transferred states relevant to magnetic phase transition in rubidium manganese hexacyanoferrate[J]. Journal of Luminescence, 2009, 129(12): 1775-1778. [18] ZHANG X J, HE P, ZHANG X Q, et al. Manganese hexacyanoferrate/multi-walled carbon nanotubes nanocomposite: Facile synthesis, characterization and application to high performance supercapacitors[J]. Electrochimica Acta, 2018, 276: 92-101. [19] WANG W W, ZHANG P L, LI S, et al. Nitrogen-doped carbon-wrapped porous FeMnO3 nanocages derived from etched Prussian blue analogues as high-performance anode for lithium ion batteries[J]. Journal of Power Sources, 2020, 475: 228683. [20] LIU P, LIU W, Huang Y, et al. Mesoporous hollow carbon spheres boosted, integrated high performance aqueous Zn-ion energy storage[J]. Energy Storage Materials, 2020, 25: 858-865. [21] CHEN B, YU J H, LU X Y, et al. Effects of reduction method on reduced graphene oxide and its electrochemical energy storage performance[J]. Diamond and Related Materials, 2021, 114: 108305. [22] LI M, SCIACCA R, MAISURADZE M, et al. Electrochemical performance of manganese hexacyanoferrate cathode material in aqueous Zn-ion battery[J]. Electrochimica Acta, 2021, 400: 139414.



 下载:
下载: